From pulsed field ablation to diabetes and surgical robotics, there are many important medical devices being launched.
1. Pulsed Field Ablation
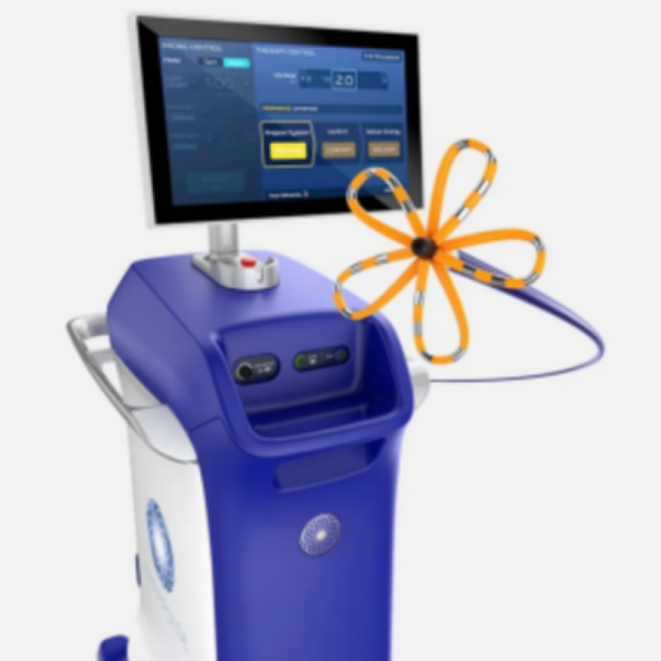
Device: Farapulse
The system was acquired by Boston Scientific in 2021 for nearly $300 million. The entire system includes the Farawave ablation catheter, Farastar ablation generator, and Faradrive steerable sheath, which together provide pulsed field energy. Farapulse uses non-thermal treatment technology to selectively ablate cardiac tissue through electric fields, and its catheter design is unique. It is an online catheter with a variable shape that can adapt to the pulmonary venous anatomy of different patients to achieve personalized treatment.
2. Tricuspid Valve Replacement
Device: Evoque Tricuspid
Evoque is a transcatheter therapy designed by Edwards Lifesciences specifically for the treatment of tricuspid regurgitation. The therapy received FDA approval in February, becoming the first FDA-approved transcatheter device for tricuspid regurgitation. Evoque features a nitinol self-expanding frame, annular skirt, and tissue leaflets made from bovine pericardial tissue.
3. Continuous Glucose Monitoring
Device: Non-insulin Designed CGM
Stelo CGM is a 15-day continuous glucose monitoring system designed by Dexcom, and the software experience is specifically customized for non-insulin dependent diabetics.
Shortly after Dexcom submitted its application to the FDA, company CEO Kevin Sayer told Drug Delivery Business News that Stelo CGM was designed with non-insulin users in mind and would not “bother” users with frequent alerts and reminders, but instead provide the personalized insights users need. As a result, Sayer noted that the company’s production line is ready for production. In addition, it is worth noting that Stelo CGM was not initially promoted as a cash payment option, but was acquired through an over-the-counter transaction in March.
4. Surgical Robotic Applications
Equipment: Mako Spine/Shoulder
Early last year, Stryker said that its Mako surgical robotic platform will achieve significant application expansion in 2024. Mako Spine is an application combined with the company’s new bone cutting product. And Mako Shoulder will use robotic applications to increase incremental precision.
In addition, Stryker will continue to expand the scope of Mako’s applications. Earlier this year, they launched a new medium to enhance the surgeon’s experience using the Mako intelligent robotic system in and outside the operating room.
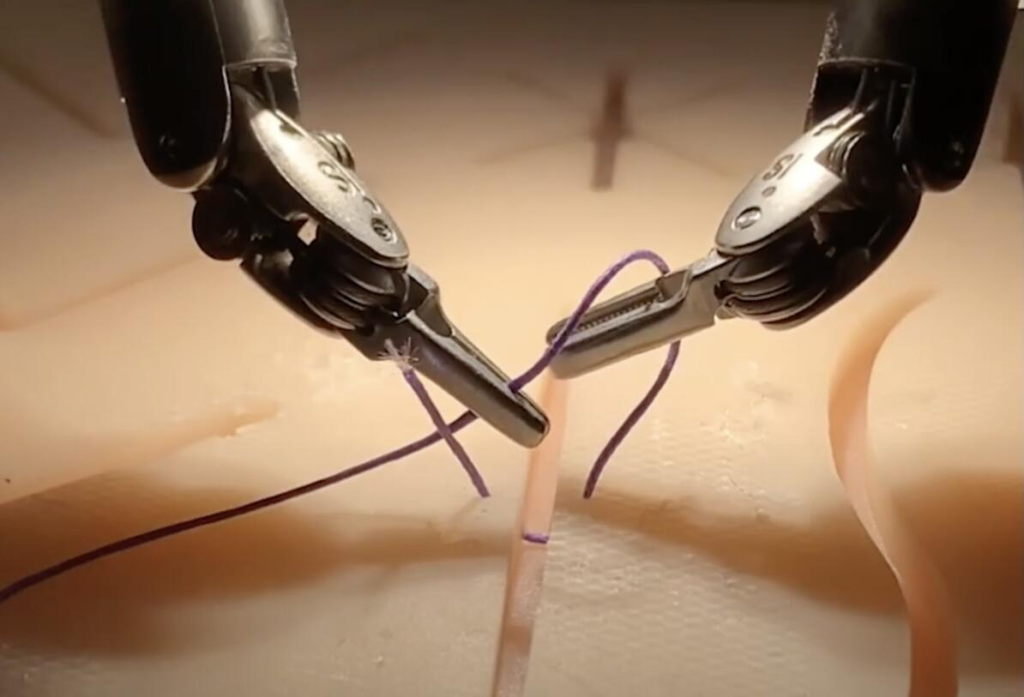
5. New Applications of ROSA Surgical Robots
Equipment: ROSA Shoulder
Zimmer Biomet’s ROSA Shoulder robotic-assisted surgical system was approved by the FDA in February. The company’s ROSA Shoulder supports surgeons in total shoulder replacement using anatomical or reverse techniques. Through precise implant positioning, ROSA Shoulder helps improve surgical outcomes. According to Zimmer Biomet, ROSA Shoulder is one of the few systems that can accurately replicate the humeral head resection process. In addition, the system avoids the use of a needle in the center of the glenoid during surgery, simplifies the steps of inserting instruments into the incision, and thus improves the convenience and safety of the surgery.
Shenzhen Yujiaxin Tech Co., Ltd. specializes in the production of various types of laparoscopic surgical instrument forceps, surgical robot grasping forceps, electrocoagulation forceps, surgical blades, surgical grasping forceps, ultrasonic surgical blades, medical equipment parts and mechanical medical equipment parts, etc. The company also provides OEM customized processing services to meet the personalized needs of customers.




